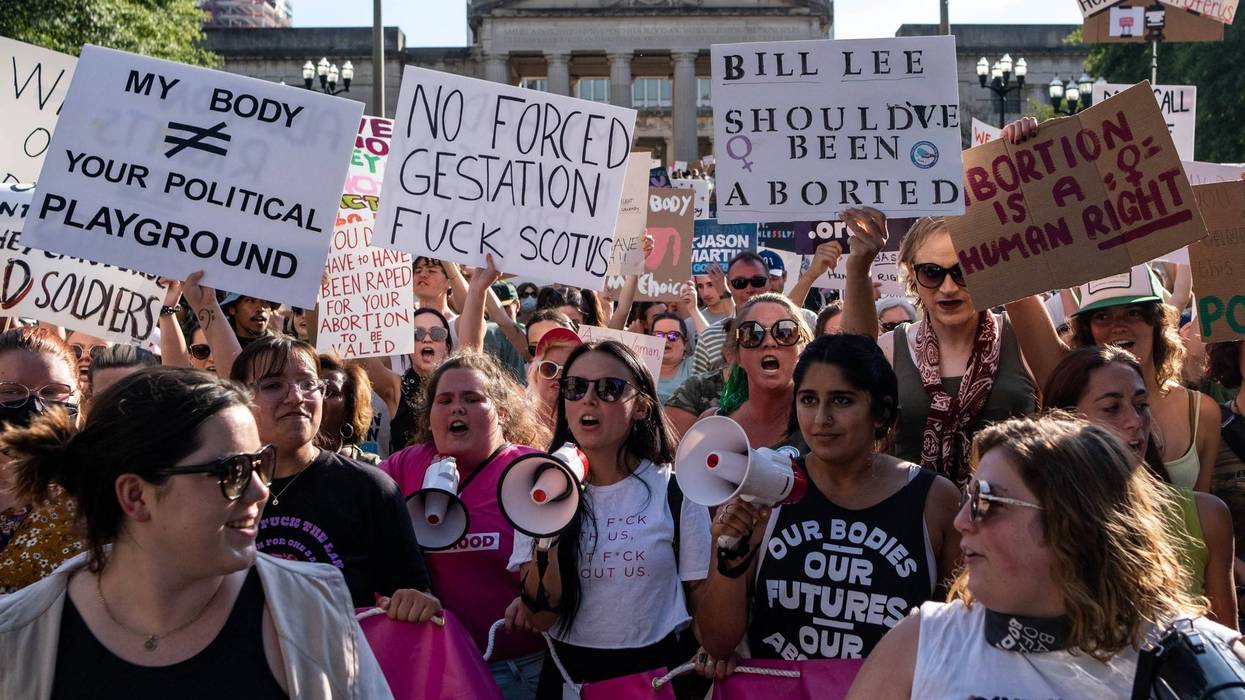October, 23 2020, 12:00am EDT

FDA's COVID-19 Vaccine Emergency Use Authorization Would Rely on Seriously Deficient Vaccination Data
Sidney Wolfe Testifies Before FDA COVID-19 Vaccine Committee
WASHINGTON
The U.S. Food and Drug Administration recently increased some requirements for granting an Emergency Use Authorization (EUA) for a COVID-19 vaccine, but serious deficiencies remain, Sidney Wolfe, founder of Public Citizen's Health Research Group, said in testimony Thursday before the agency's Vaccines and Related Biological Products Advisory Committee. The hearing followed a protest outside of the U.S. Department of Health and Human Services building where activists demanded a safe, effective, accessible and free COVID-19 vaccine.
Public confidence in a COVID-19 vaccine continues to wane, and rushing a vaccine through an EUA without complete Phase 3 safety and efficacy data, which could take months to complete, would further undermine public confidence.
Wolfe asked the committee, "Based on incomplete Phase 3 trials, will your advisory committee have enough confidence, despite all this missing data, to recommend authorizing a vaccine, for use in tens of millions of people, when an EUA is requested? The gap between completed Phase 3 trials needed for approval and the current EUA standard, allowing half of Phase 3 trial participants to be followed for less than two months after vaccination does not engender confidence. Saving time by a faster but riskier data-deficient EUA pathway will surely be outweighed by further loss in public confidence in an incompletely tested, vaccine, accompanied by decreased willingness to be vaccinated."
Public Citizen is a nonprofit consumer advocacy organization that champions the public interest in the halls of power. We defend democracy, resist corporate power and work to ensure that government works for the people - not for big corporations. Founded in 1971, we now have 500,000 members and supporters throughout the country.
(202) 588-1000LATEST NEWS
Republican Lawmakers' Bid to Execute Tennessee Abortion Patients Slammed as 'Christofascism'
"This is about the future of the anti-abortion movement in the Republican Party and the way that they are embracing extremism at a rate that is so fucking alarming," said one critic.
Feb 23, 2026
“If you kill a baby from embryo on up with a pill or a scalpel, we oughta execute you."
That's not social media rage bait by some random zealot, it's the premise of legislation recently introduced by Republican state lawmakers in Tennessee to make abortion a capital offense, as voiced by one of the measure's sponsors. And it's setting off alarm bells in recent days across a nation in which attacks on remaining reproductive rights have been accelerating in the years since the right-wing US Supreme Court overturned its landmark Roe v. Wade ruling nearly four years ago.
An amendment to HB 570/SB 738 was filed by primary sponsors Rep. Jody Barrett (R-69) and Sen. Mark Pody (R-17) and co-sponsored by five of their GOP colleagues, all men, including Rep. Monty Fritts (R-32), who is also running for governor—and who is the source of the quote in this article's lede. Fritts spoke those words at a meeting in Jonesborough, where TN Repro News publisher Rachel Wells last year interviewed a pregnant woman who was allegedly denied prenatal care under Tennessee's Medical Ethics Defense Act because she is unmarried to her partner of 15 years.
If passed, Barrett and Pody's amendment—which was still adding co-sponsors as of Monday—would classify abortion as "homicide of an unborn child," punishable by life imprisonment with or without parole—or even death by lethal injection. The measure contains very narrow exceptions, including for spontaneous miscarriage or when abortion is needed to save a mother's life. The amendment is currently under committee review has not yet been scheduled for a vote.
Tennessee already has some of the strictest abortion laws in the United States, with a near-total ban on the procedure in effect since Republican Gov. Bill Lee signed it in August 2022. Abortion is banned from fertilization, with limited exceptions.
While religious groups including the Southern Baptist Convention and Foundation to Abolish Abortion hailed the proposal as a life-saving measure that serves the will of the Abrahamic deity figure "God," reproductive rights defenders expressed alarm and outrage.
"We are talking about a gubernatorial candidate openly calling for women who end their pregnancies to be charged with a capital crime and spend their life in prison or for the to get the death penalty. That is where we're at right now," Abortion, Every Day publisher Jessica Valenti said in a video posted on social media.
"This is not just about this one guy," she continued. "This is about the future of the anti-abortion movement in the Republican Party and the way that they are embracing extremism at a rate that is so fucking alarming."
Meet Rep. Monty Fritts— a Tennessee lawmaker running for governor. If you’re one of the millions of American women who’s had an abortion, he thinks that you should be given the death penalty
[image or embed]
— Jessica Valenti (@jessicavalenti.bsky.social) February 18, 2026 at 7:57 PM
"Saying that women should be punished for having abortions was once... an unthinkable thing to say within the anti-abortion movement," Valenti added. "Now they're openly embracing it. Over a dozen states over the last year have introduced or advanced equal protection legislation... that would punish abortion patients as murders, which in some states can mean the death penalty, it could mean life in prison."
"This is not some fringe element," she stressed. "This is becoming the mainstream of the movement. Right now in Texas... the Republican Party platform calls for equal protection. It calls for the execution of women or life in prison for women who have abortions. This is not fringe."
In South Carolina, where a bill to execute people who have abortions garnered more than 20 GOP votes on its way to defeat but performing the procedure is a felony, the Sumter County Sheriff's Office last week launched an investigation into a fetus that was found at a water treatment plant. Investigators will test tissue samples from the fetus "to determine the race and locate the mother."
Numerous deaths have been attributed to abortion bans in states including Texas and Georgia.
Back in Tennessee, Fritts—who is polling at around 5-7% in the GOP gubernatorial primary, depending on the survey—has been busy defending his proposal to kill people who have abortions.
“Murder is murder. I know that’s hard for people to hear, and I don’t mean to be hard with it, I promise,” he told the Tennessee Holler, comparing abortion pills to cyanide capsules.
Fritts' campaign slogan is "liberty & less government."
Responding to Fritts' co-sponsorship of the death penalty amendment, Jon Tate's Daily Practice publisher Jon Tate wrote, "Disgusting."
"While I was busy and not paying attention, my state was apparently becoming ground zero for white-supremacist Christofascism," he added. "It breaks my brain and my heart."
Keep ReadingShow Less
Huckabee Accused of Inciting Murder After Israeli Settlers Kill Palestinian-American Teen
"The US ambassador to Israel is engaging in empowering and allowing for actions that lead to the targeted lynching and killing of US citizens," said one group.
Feb 23, 2026
Human rights defenders this week accused US Ambassador to Israel Mike Huckabee—who recently endorsed Israel conquering much of the Middle East—of inciting deadly violence after Israeli colonists in the illegally occupied West Bank of Palestine fatally shot a Palestinian-American teenager who was trying to stop settlers from stealing livestock.
Nasrallah Abu Siyam, 19, was shot dead last Wednesday by a masked Israeli settler armed with an M-16 rifle in the village of Mukhmas, where the 19-year-old Philadelphia native had been living and helping his father, Mohammed Abu Siyam, tend the family's livestock and cultivate their olive trees.
According to eyewitness accounts as reported by independent New York journalist and Palestine specialist Jasper Diamond Nathaniel:
At least four other local Palestinians were wounded by settler gunfire during the invasion of the village, including another young man whose foot may be amputated. Some were shot while carrying the wounded to safety. Many others were severely beaten with metal rods. Israeli soldiers, who accompanied the settlers into the village, responded to the shooting rampage by firing stun grenades and tear gas into the residential area, burning an elderly man. When it was over, settlers walked off with more than 300 of the village’s sheep and goats under the military’s watch. It was the first full day of Ramadan. As of this writing, no one has been arrested.
While human rights groups and some Democratic US lawmakers have called for a full investigation into Abu Siyam's killing, Huckabee has so far been silent. Last July, Huckabee responded to Israeli settlers' killing of 23-year-old Palestinian-American Sayfollah Musallet, who was beaten to death while visiting relatives in the West Bank, as "a criminal and terrorist act" that Israeli authorities should "aggressively investigate." As is usually the case when Israeli settlers kill Palestinians, no one has been charged for killing Musallet.
Last Friday, Huckabee—who during his ill-fated 2008 presidential campaign denied the very existence of the Palestinian people—sat for an interview with conservative commentator Tucker Carlson during which he backed the realization of a so-called “Greater Israel” stretching from the Nile River in Egypt to the Euphrates in Iraq, saying that "it would be fine" if Israel "took it all," as many Jews and Evangelical Christians believe their common deity figure "God" intended them to do.
Numerous observers said the envoy's remarks inherently endorsed violence and forced displacement akin to what's happening to Palestinians living under occupation, colonization, ethnic cleansing, apartheid—and in the case of Gaza, genocide.
"Shortly after the lynching murder of an American citizen, footage aired of the US Ambassador to Israel Mike Huckabee justifying the very structure of occupation, and rhetoric of ethnic cleansing, that led to the murder and continuing attacks on the occupied West Bank," the American-Arab Anti-Discrimination Committee (ADC) said in a statement Monday.
ADC said Huckabee's endorsement of Greater Israel "signals permission and the green light for Israeli forces to use violence and empower settlers for further annexation and dispossession."
The group continued:
The United States continues to fund, shield, and excuse Israeli violence, forced displacement, and mass atrocity across Palestine. Now the US ambassador to Israel is engaging in empowering and allowing for actions that lead to the targeted lynching and killing of US citizens. At the same time, Congress continues to put Israel first by sending American taxpayer dollars to Israel.
Israeli settlers and soldiers have killed at least a dozen Americans since 2022. Time and again, our government refuses to defend the rights, dignity, and safety of its own citizens simply to appease the demands of a foreign government and give impunity to Israel.
"The impunity cannot continue," ADC added.
Israeli leaders including Prime Minister Benjamin Netanyahu—a fugitive from the International Criminal Court wanted for alleged crimes against humanity and war crimes in Gaza—have publicly declared their support for Greater Israel, sparking widespread condemnation throughout the Arab world and beyond.
Keep ReadingShow Less
Polio Survivors Fear a Resurgence as Trump Adviser Suggests Scrapping Vaccine Requirement
"We don’t have a healthcare infrastructure to take care of a polio outbreak."
Feb 23, 2026
After the Trump administration official in charge of immunization policy suggested that childhood polio vaccines should be made optional, experts and survivors of the deadly disease are warning that it could make a furious comeback.
Dr. Kirk Milhoan, a pediatric cardiologist who is chair of the Advisory Committee on Immunization Practices, suggested on a podcast last month ending public schools' vaccine requirements for dangerous diseases, including measles and polio, which would be one of the most dramatic shifts in federal health policy in more than half a century.
Where these diseases once infected millions of people each year, Milhoan noted their dramatic decline in recent years, suggesting they no longer pose the threat they once did and that vaccines were therefore less necessary. However, he ignored the fact that the near-total eradication of these illnesses was due to society-wide vaccination in the first place.
In the first half of the 20th century, tens of thousands of people (mostly children) suffered paralysis from polio. The first vaccine was introduced in the USA in 1955. Notice the trend afterwards.(by @ourworldindata)
[image or embed]
— Information is Beautiful (@infobeautiful.bsky.social) January 26, 2026 at 2:55 PM
The US is already at risk of losing its measles eradication status after drops in vaccination rates caused the highest number of cases and deaths in more than three decades last year.
Measles vaccination rates had already been dipping for years amid rising anti-vaccine sentiment. But it was shifted into overdrive after vaccination restrictions were narrowed by Trump's Secretary of Health and Human Services, Robert F. Kennedy Jr., who publicly spread doubt about the Measles, Mumps, and Rubella (MMR) vaccine's well-documented safety and efficacy.
At its peak in 1952, nearly 58,000 people became infected with polio. Over a third of them became paralyzed, and more than 3,000 died. A vaccine was introduced for the illness in 1955. Within just two years, the number of cases had dropped by 90%, and the disease was declared eliminated in the US in 1979.
Childhood vaccination rates have dropped across the board over the past five years. Where about 95% of kindergarteners received the measles and polio vaccines in the 2019-20 school year, that number had plummeted to 92.5% in 2024-25.
But because polio is several times less infectious than measles, the current national average coverage still provides substantial protection, though localized outbreaks remain possible in undervaccinated communities.
If childhood vaccination is made optional, however, those who have treated and lived with polio fear it could also come back with a vengeance.
Survivors say US healthcare system not ready for new cases – ‘the only thing to fix polio is the polio vaccine’
[image or embed]
— Guardian US (@us.theguardian.com) February 23, 2026 at 2:13 PM
In an interview with the Guardian published Monday, Grace Rossow, an operating-room communications coordinator, whose leg remains paralyzed from a case of polio as an infant in India, said the vaccine had “absolutely been a victim of its own success."
“People aren’t scared of polio anymore,” she said. “People don’t really see the daily side of living with a vaccine-preventable disease. With polio, you’re never going to fix us, and that’s the problem. The only thing to fix polio is the polio vaccine.”
Polio's status as a thing of the past has not only diminished the public's understanding of why it's important to prevent, but also how to treat it. Rossow said, "We don’t have a healthcare infrastructure to take care of a polio outbreak."
Art Caplan, one of the last remaining survivors of the 1950s outbreak, who has struggled with lifelong weakness in his legs due to the disease, said he's watched as most of the medical professionals who understood how to treat it retired and died. "There’s nobody left. They don’t see it."
Gordon Allan, a surgeon who is the orthopedic residency director and the total joint reconstructive fellowship director at Southern Illinois University School of Medicine, said that in the event of a new polio outbreak, most people in his field would have little idea how to treat those suffering from the illness.
“Orthopedics has really changed a lot now from the people who trained me," he said, noting that even those doctors only had experience treating post-polio symptoms.
“No one practicing has first-hand experience," he said. "Orthopedics was quite different because of polio, and all that stuff just faded away."
The last polio case in the United States was detected in New York in 2022 in an unvaccinated adult who became paralyzed from the illness.
None have been diagnosed since. But as measles has shown, outbreaks can spread very quickly in communities with large unvaccinated populations, which are often insular and religious.
Caplan said he was "furious" at Milhoan's contention that childhood vaccine requirements should be reconsidered just because polio is no longer around.
"If you could gather up the kids I saw die or become really severely disabled from 50 years ago, they would want you arrested," he said. "It’s horrifying, and the height of irresponsibility to leave the door open even a crack."
Keep ReadingShow Less
Most Popular