November, 11 2009, 03:06pm EDT

Medicare Should Deny Payment for Unproven Anti-Depression Device
Pre-Market Testing Was Flawed, Public Citizen says
WASHINGTON
Medicare should deny reimbursement for a medical device for
depression that the Food and Drug Administration (FDA) approved despite
unconvincing test results, Public Citizen said today in a letter sent to Medicare officials.
"The scant evidence presented for approval of this device does not
come close to matching the type of data typically required for approval
of anti-depressant drugs" said Dr. Peter Lurie, deputy director of
Public Citizen's Health Research Group. "Medicare should not approve
coverage of this unproven device because it could divert patients from
more effective therapies."
The controlled trial submitted to the FDA for approval of
Neuronetics' NeuroStar Transcranial Magnetic Stimulation Therapy System
showed that the costly device had no significant clinical or
statistical impact in treating major depressive disorder in patients
who had not benefited from prior antidepressant medication, Public
Citizen said.
Public Citizen's letter to the Centers for Medicare and Medicaid
Services requests that the agency issue a negative "national coverage
determination," which would preclude the agency for reimbursing
providers who use the device, saying the device has never met FDA's
effectiveness standard for approval, much less Medicare's more
stringent standard that the device be "reasonable and necessary."
Neuronetics' device directs short, alternating bursts of magnetic
impulses to the nerve cells in the part of the brain associated with
mood regulation. The device initially went through the FDA's less
rigorous 510(k) approval process because Neuronetics claimed that the
NeuroStar device was similar to another device the FDA had already
approved. Ultimately it was approved through a relatively obscure
pathway called de novo.
But Public Citizen's letter cites the exclusion of six patients from
NeuroStar's controlled trial and statistical manipulations after the
trial was completed that skewed the results in the company's favor.
READ Public Citizen's letter.
Public Citizen is a nonprofit consumer advocacy organization that champions the public interest in the halls of power. We defend democracy, resist corporate power and work to ensure that government works for the people - not for big corporations. Founded in 1971, we now have 500,000 members and supporters throughout the country.
(202) 588-1000LATEST NEWS
'We Cannot Be Silent': Tlaib Leads 19 US Lawmakers Demanding Israel Stop Starving Gaza
"This current blockade is starving Palestinian civilians in violation of international law, and the militarization of food will not help."
Jun 30, 2025
As the death toll from Israel's forced starvation of Palestinians continues to rise amid the ongoing U.S.-backed genocidal assault and siege of the Gaza Strip, Rep. Rashida Tlaib on Monday led 18 congressional colleagues in a letter demanding that the Trump administration push for an immediate cease-fire, an end to the Israeli blockade, and a resumption of humanitarian aid into the embattled coastal enclave.
"We are outraged at the weaponization of humanitarian aid and escalating use of starvation as a weapon of war by the Israeli government against the Palestinian people in Gaza," Tlaib (D-Mich.)—the only Palestinian American member of Congress—and the other lawmakers wrote in their letter to U.S. Secretary of State Marco Rubio. "For over three months, Israeli authorities have blocked nearly all humanitarian aid from entering Gaza, fueling mass starvation and suffering among over 2 million people. This follows over 600 days of bombardment, destruction, and forced displacement, and nearly two decades of siege."
"According to experts, 100% of the population is now at risk of famine, and nearly half a million civilians, most of them children, are facing 'catastrophic' conditions of 'starvation, death, destitution, and extremely critical acute malnutrition levels,'" the legislators noted. "These actions are a direct violation of both U.S. and international humanitarian law, with devastating human consequences."
Gaza officials have reported that hundreds of Palestinians—including at least 66 children—have died in Gaza from malnutrition and lack of medicine since Israel ratcheted up its siege in early March. Earlier this month, the United Nations Children's Fund warned that childhood malnutrition was "rising at an alarming rate," with 5,119 children under the age of 5 treated for the life-threatening condition in May alone. Of those treated children, 636 were diagnosed with severe acute malnutrition, the most lethal form of the condition.
Meanwhile, nearly 600 Palestinians have been killed and more than 4,000 others have been injured as Israeli occupation forces carry out near-daily massacres of desperate people seeking food and other humanitarian aid at or near distribution sites run by the U.S.-backed Gaza Humanitarian Foundation (GHF). Israel Defense Forces officers and troops have said that they were ordered to shoot and shell aid-seeking Gazans, even when they posed no threat.
"This is not aid," the lawmakers' letter argues. "UNRWA Commissioner-General Philippe Lazzarini has warned that, under the GHF, 'aid distribution has become a death trap.' We cannot allow this to continue."
"We strongly oppose any efforts to dismantle the existing U.N.-led humanitarian coordination system in Gaza, which is ready to resume operations immediately once the blockade is lifted," the legislators wrote. "Replacing this system with the GHF further restricts lifesaving aid and undermines the work of long-standing, trusted humanitarian organizations. The result of this policy will be continued starvation and famine."
"We cannot be silent. This current blockade is starving Palestinian civilians in violation of international law, and the militarization of food will not help," the lawmakers added. "We demand an immediate end to the blockade, an immediate resumption of unfettered humanitarian aid entry into Gaza, the restoration of U.S. funding to UNRWA, and an immediate and lasting cease-fire. Any other path forward is a path toward greater hunger, famine, and death."
Since launching the retaliatory annihilation of Gaza in response to the Hamas-led October 7, 2023 attack on Israel, Israeli forces have killed at least 56,531 Palestinians and wounded more than 133,600 others, according to the Gaza Health Ministry, which also says over 14,000 people are missing and presumed dead and buried beneath rubble. Upward of 2 million Gazans have been forcibly displaced, often more than once.
On Sunday, U.S. President Donald Trump reiterated a call for a cease-fire deal that would secure the release of the remaining 22 living Israeli and other hostages held by Hamas.
In addition to Tlaib, the letter to Rubio was signed by Sen. Bernie Sanders (I-Vt.) and Democratic Reps. Greg Casar (Texas), Jesús "Chuy" García (Ill.), Al Green (Texas), Jonathan Jackson (Ill.), Pramila Jayapal (Wash.), Henry "Hank"Johnson (Ga.), Summer Lee (Pa.), Jim McGovern (Mass.), Alexandria Ocasio-Cortez (N.Y.), Ilhan Omar (Minn.), Chellie Pingree (Maine), Mark Pocan (Wisc.), Ayanna Pressley (Mass.), Delia Ramirez (Ill.), Paul Tonko (N.Y.), Nydia Velázquez (N.Y.), and Bonnie Watson Coleman (N.J.).
Keep ReadingShow Less
Biden National Security Adviser Among Those Crafting 'Project 2029' Policy Agenda for Democrats
"Jake Sullivan's been a critical decision-maker in every Democratic catastrophe of the last decade," said one observer. "Why is he still in the inner circle?"
Jun 30, 2025
Amid the latest battle over the direction the Democratic Party should move in, a number of strategists and political advisers from across the center-left's ideological spectrum are assembling a committee to determine the policy agenda they hope will be taken up by a Democratic successor to President Donald Trump.
Some of the names on the list of people crafting the agenda—named Project 2029, an echo of the far-right Project 2025 blueprint Trump is currently enacting—left progressives with deepened concerns that party insiders have "learnt nothing" and "forgotten nothing" from the president's electoral victories against centrist Democratic candidates over the past decade, as one economist said.
The project is being assembled by former Democratic speechwriter Andrei Cherny, now co-founder of the policy journal Democracy: A Journal of Ideas, and includes Jake Sullivan, a former national security adviser under the Biden administration; Jim Kessler, founder of the centrist think tank Third Way; and Neera Tanden, president of the Center for American Progress and longtime adviser to former Secretary of State Hillary Clinton.
Progressives on the advisory board for the project include economist Justin Wolfers and former Roosevelt Institute president Felicia Wong, but antitrust expert Hal Singer said any policy agenda aimed at securing a Democratic victory in the 2028 election "needs way more progressives."
As The New York Times noted in its reporting on Project 2029, the panel is being convened amid extensive infighting regarding how the Democratic Party can win back control of the White House and Congress.
After democratic socialist and state Assemblymember Zohran Mamdani's (D-36) surprise win against former New York Gov. Andrew Cuomo last week in New York City's mayoral primary election—following a campaign with a clear-eyed focus on making childcare, rent, public transit, and groceries more affordable—New York City has emerged as a battleground in the fight. Influential Democrats including House Minority Leader Hakeem Jeffries (D-N.Y.) and Sen. Kirsten Gillibrand (D-N.Y.) have so far refused to endorse him and attacked him for his unequivocal support for Palestinian rights.
Progressives have called on party leaders to back Mamdani, pointing to his popularity with young voters, and accept that his clear message about making life more affordable for working families resonated with Democratic constituents.
But speaking to the Times, Democratic pollster Celinda Lake exemplified how many of the party's strategists have insisted that candidates only need to package their messages to voters differently—not change the messages to match the political priorities of Mamdani and other popular progressives like Sen. Bernie Sanders (I-Vt.) and Rep. Alexandria Ocasio-Cortez (D-N.Y.).
"We didn't lack policies," Lake told the Times of recent national elections. "But we lacked a functioning narrative to communicate those policies."
Sanders and Ocasio-Cortez have drawn crowds of thousands in red districts this year at Sanders' Fighting Oligarchy rallies—another sign, progressives say, that voters are responding to politicians who focus on billionaires' outsized control over the U.S. political system and on economic justice.
Project 2029's inclusion of strategists like Kessler, who declared economic populism "a dead end for Democrats" in 2013, demonstrates "the whole problem [with Democratic leadership] in a nutshell," said Jonathan Cohn of Progressive Mass—as does Sullivan's seat on the advisory board.
As national security adviser to President Joe Biden, Sullivan played a key role in the administration's defense and funding of Israel's assault on Gaza, which international experts and human rights groups have said is a genocide.
"Jake Sullivan's been a critical decision-maker in every Democratic catastrophe of the last decade: Hillary Clinton's 2016 campaign, the withdrawal from Afghanistan, the Israel/Gaza War, and the 2024 Joe Biden campaign," said Nick Field of the Pennsylvania Capital-Star. "Why is he still in the inner circle?"
"Jake Sullivan is shaping domestic policy for the next Democratic administration," he added. "Who is happy with the Biden foreign policy legacy?"
Keep ReadingShow Less
Rick Scott Pushes Amendment to GOP Budget Bill That Could Kick Millions More Off Medicaid
Scott's proposal for more draconian cuts has renewed scrutiny regarding his past as a hospital executive, where he oversaw the "largest government fraud settlement ever," which included stealing from Medicaid.
Jun 30, 2025
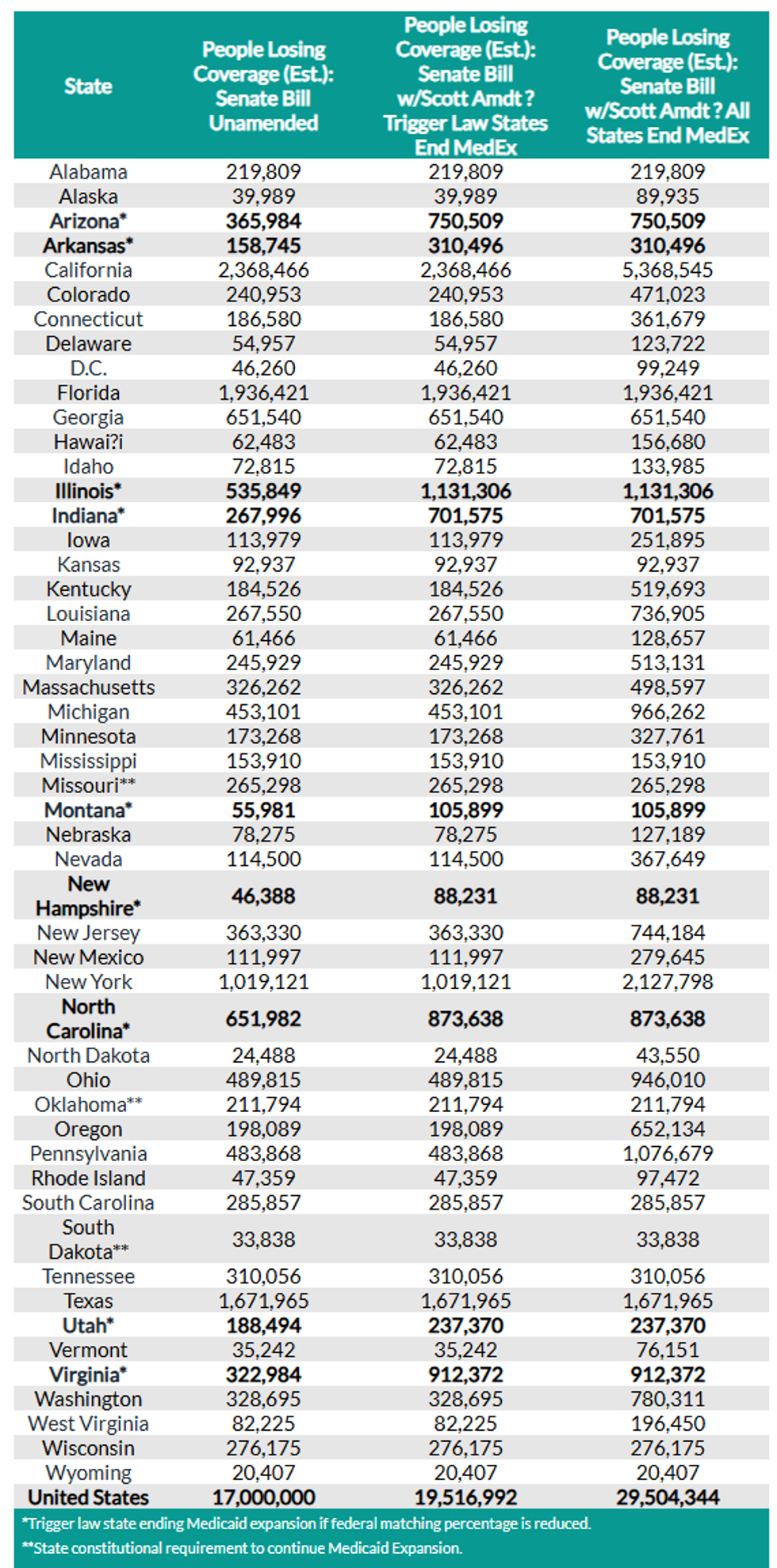
Sen. Rick Scott has introduced an amendment to the Republican budget bill that would slash another $313 million from Medicaid and kick off millions more recipients.
The latest analysis by the Congressional Budget Office (CBO) found that 17 million people could lose their health insurance by 2034 as the result of the bill as it already exists.
According to a preliminary estimate by the Democrats on the Joint Congressional Economic Committee, that number could balloon up to anywhere from 20 to 29 million if Scott's (R-Fla.) amendment passes.
The amendment will be voted on as part of the Senate's vote-a-rama, which is expected to run deep into Monday night and possibly into Tuesday morning.
"If Sen. Rick Scott's amendment gets put forward, this would be a self-inflicted healthcare crisis," said Tahra Hoops, director of economic analysis at Chamber of Progress.
The existing GOP reconciliation package contains onerous new restrictions, including new work requirements and administrative hurdles, that will make it harder for poor recipients to claim Medicaid benefits.
Scott's amendment targets funding for the program by ending the federal government's 90% cost sharing for recipients who join Medicaid after 2030. Those who enroll after that date would have their medical care reimbursed by the federal government at a lower rate of 50%.
The Affordable Care Act (ACA) introduced the increased rate in 2010 to incentivize states to expand Medicaid, allowing more people to be covered.
Scott has said his program would "grandfather" in those who had already been receiving the 90% reimbursement rate.
However, Medicaid is run through the states, which will have to spend more money to keep covering those who need the program after 2030.
The Center on Budget and Policy Priorities estimated that this provision "would shift an additional $93 billion in federal Medicaid funding to states from 2031 through 2034 on top of the cuts already in the Senate bill."
This will almost certainly result in states having to cut back, by introducing their stricter requirements or paperwork hurdles.
Additionally, nine states have "trigger laws" that are set to end the program immediately if the federal matching rate is reduced: Arizona, Arkansas, Illinois, Indiana, Montana, New Hampshire, North Carolina, Utah, and Virginia.
The Joint Congressional Economic Committee estimated Tuesday that around 2.5 million more people will lose their insurance as a result of those cuts.
If all the states with statutory Medicaid expansion ended it as a result of Scott's cuts, as many as 12.5 million could lose their insurance. Combined with the rest of the bill, that's potentially 29 million people losing health insurance coverage, the committee said.
There are enough Republicans in the Senate to pass the bill with Scott's amendment. However, they can afford no more than three defections. According to Politico, Sens. Rand Paul (R-Ky.) and Thom Tillis (R-N.C.) have signaled they will vote against the amendment.
Sen. Jim Justice (R-W.V.) also said he'd "have a hard time" voting yes on the bill if Scott's amendment passed. His state of West Virginia has the second-highest rate of people using federal medical assistance of any state in the country, behind only Mississippi.
Critics have called out Scott for lying to justify this line of cuts. In a recent Fox News appearance, Scott claimed that his new restrictions were necessary to stop Democrats who want to "give illegal aliens Medicaid benefits," even though they are not eligible for the program.
Scott's proposal has also brought renewed scrutiny to his past as a healthcare executive.
"Ironically enough, some of the claims against Scott's old hospital company revolved around exploiting Medicaid, and billing for services that patients didn't need," wrote Andrew Perez in Rolling Stone Monday.
In 2000, Scott's hospital company, HCA, was forced to pay $840 million in fines, penalties, and damages to resolve claims of unlawful billing practices in what was called the "largest government fraud settlement ever." Among the charges were that during Scott's tenure, the company overbilled Medicare and Medicaid by pretending patients were sicker than they actually were.
The company entered an additional settlement in 2003, paying out another $631 million to compensate for the money stolen from these and other government programs.
Scott himself was never criminally charged, but resigned in 1997 as the Department of Justice began to probe his company's activities. Despite the scandal, Scott not only became a U.S. senator, but is the wealthiest man in Congress, with a net worth of more than half a billion dollars.
The irony of this was not lost on Perez, who wrote: "A few decades later, Scott is now trying to extract a huge amount of money from state Medicaid funds to help finance Trump's latest round of tax cuts for the rich."
Keep ReadingShow Less
Most Popular


