

SUBSCRIBE TO OUR FREE NEWSLETTER
Daily news & progressive opinion—funded by the people, not the corporations—delivered straight to your inbox.
5
#000000
#FFFFFF
To donate by check, phone, or other method, see our More Ways to Give page.


Daily news & progressive opinion—funded by the people, not the corporations—delivered straight to your inbox.
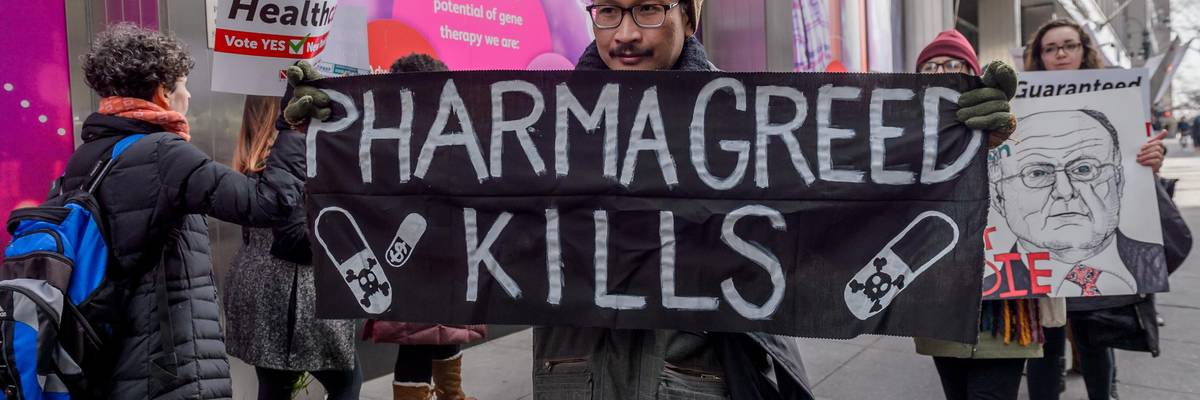
Medical professionals, students, ACTUP New York, and their supporters protested outside Pfizer's global headquarters in New York City on March 2, 2019.
"We request HHS to consider this appeal directly... because the NIH has repeatedly demonstrated its unwillingness to even acknowledge that the Bayh-Dole Act includes an obligation to make products invented with federal funds 'available to the public on reasonable terms.'"
Two days after President Joe Biden's administration rejected a petition asking federal regulators to use their authority to lower the astronomical price of a lifesaving prostate cancer drug developed entirely with public funds, petitioners on Thursday filed an administrative appeal.
At issue is enzalutamide, a drug the Japanese pharmaceutical giant Astellas and its U.S. counterpart Pfizer sell under the brand name Xtandi. Although Xtandi owes its existence to U.S. taxpayers, who bankrolled 100% of its development, an annual supply of the drug costs $189,900 in the United States—three to six times more than its list price in other wealthy nations.
In late 2021, prostate cancer patients Robert Sachs, Clare Love, and Eric Sawyer petitioned the U.S. Department of Health and Human Services (HHS) to exercise its "march-in rights" against Xtandi. Under the Bayh-Dole Act, the federal government can reclaim and redistribute patents for inventions created with public funding—enabling generic competitors to produce cheaper versions—when "action is necessary to alleviate health or safety needs" or when an invention's benefits are not being made "available to the public on reasonable terms."
HHS Secretary Xavier Becerra referred the petition to the National Institutes of Health (NIH), whose acting Director Lawrence Tabak argued in a Tuesday letter that "Xtandi is widely available to the public on the market," citing Astellas' estimate that "more than 200,000 patients were treated with Xtandi from 2012 to 2021."
Even with insurance, co-pays for Xtandi are sky-high. Medicare recipients, for example, are expected to pay roughly $10,000 per year for the medicine. Especially for the millions of uninsured and underinsured people in the U.S., Xtandi remains completely out of reach.
Tabak's letter went on to say that Xtandi's "practical application is evidenced by the 'manufacture, practice, and operation' of the invention and the invention's 'availability to and use by the public….'" As Knowledge Ecology International executive director James Love lamented, the NIH completely elided any mention of "reasonable terms," editing out that key phrase from Bayh-Dole.
In their appeal, the petitioners wrote: "The petition focused on a single issue: the reasonableness of charging U.S. cancer patients three to six times more than residents of other high-income countries for the drug Xtandi."
"There is no dispute about the following facts," the appeal continues. "Xtandi was invented on grants from the U.S. Army and the NIH at UCLA, a public university. The patents were licensed eventually to Astellas, a Japanese drug company, with a partnership share now held by Pfizer, following its 2016 $14 billion acquisition of Medivation, UCLA's original licensee, that occurred just after the NIH rejected an earlier march-in request on Xtandi. The prices in the United States have consistently been far higher than the prices in other high-income countries."
Prior to the 2021 petition, Clare Love and prostate cancer patient David Reed filed a petition, later joined by Sachs, with the U.S. Department of Defense (DOD) after the Senate Armed Services Committee instructed the Pentagon to initiate march-in proceedings when the price of a drug created with a DOD grant exceeds the median price in seven large high-income nations. The Pentagon, however, has yet to acknowledge or act on the petition submitted to it in February 2019.
"If you consider both of these requests together, a petition to exercise the government's march-in or other rights in the Xtandi patents has been pending before the federal government for more than four years," Thursday's appeal states. "The HHS petition was filed 16 months ago."
It continues:
The petitions were filed with the DOD and HHS instead of the NIH because the NIH has repeatedly demonstrated its unwillingness to even acknowledge that the Bayh-Dole Act includes an obligation to make products invented with federal funds 'available to the public on reasonable terms.' This is demonstrated by a track record of dismissing multiple requests to use the government's Bayh-Dole safeguard to address pricing abuses and access restrictions, including those concerning the federal government's march-in rights under 35 USC § 203, and the federal government's global royalty-free license, under 35 USC § 202(c)(4). There are also extensive email records between Mark Rohrbaugh, currently NIH special adviser for technology transfer who is a long-time agency official, and lobbyists for drug companies and university rights holders, obtained through Freedom of Information Act requests, which not only express opposition to any safeguards regarding unreasonable pricing but organize public relations efforts against using a march-in request to address the pricing of products.
"HHS chose to assign to the NIH the evaluation of our petition regarding Xtandi," says the appeal. "We request HHS to consider this appeal directly, and not assign NIH to review its own decision. The latter would be tantamount to no review at all."
Since Bayh-Dole was enacted in 1980, "march-in rights have never been used... and NIH has repeatedly rejected the idea that affordability is a reasonable term," The American Prospect reported Wednesday. With Xtandi, "advocates thought they found the perfect test case for a new administration that paid lip service to lowering prescription drug costs."
As The Lever noted on Wednesday, the NIH's decision this week was consistent with Biden's track record:
Biden was vice president when the Obama administration rejected congressional Democrats' demand that the government use the same power to lower the skyrocketing prices of medicine in America.
As a senator in 2000, Biden was one of just eight Democrats who helped pharmaceutical lobbyists kill a measure spearheaded by Sen. Paul Wellstone (D-Minn.) and then-Rep. Bernie Sanders (I-Vt.) that would have reinstated the Reagan-era requirement that drug companies sell medicines developed with public money at a reasonable price.
That requirement was repealed by the Clinton administration in 1995, following pressure by drugmakers.
But Becerra's acquiescence to Big Pharma was more surprising. Prior to joining the Biden administration, the HHS secretary had expressed support for wielding the executive branch's authority to rein in soaring drug prices.
As the attorney general of California in the summer of 2020, "Becerra demanded the Trump administration use existing law to lower the price of medicines that were originally developed at taxpayer expense," The Lever reported. "As a member of Congress in 2016, Becerra signed on to a letter to the Obama Department of Health and Human Services calling on officials to broadly use 'march-in rights' to lower the cost of prescription drugs—including 'specialty drugs, like those to treat cancer, which are frequently developed with taxpayer funds.'"
Despite pressure from numerous members of Congress and medicine affordability advocacy groups, the NIH declared Tuesday that it "does not believe that use of the march-in authority would be an effective means of lowering the price of the drug."
Instead, the agency vowed to "pursue a whole-of-government approach informed by public input to ensure the use of march-in authority is consistent with the policy and objective of the Bayh-Dole Act," a move that progressive advocates denounced as a "pathetic" attempt to deflect criticism of its failure to use or threaten to use its legal power.
“This is a drug that was invented with taxpayer dollars by scientists at UCLA and can be purchased in Canada for one-fifth the U.S. price," Sanders said Tuesday. "The Japanese drugmaker Astellas, which made $1 billion in profits in 2021, has raised the price of this drug by more than 75%."
"How many prostate cancer patients will die because they cannot afford this unacceptable price?" asked Sanders, chair of the Senate Committee on Health, Education, Labor, and Pensions.
During a Wednesday hearing, Sanders made the case for changing "the current culture of greed into a culture which understands that science and medical breakthroughs should work for ordinary people, and not just enrich large corporations and CEOs."
Dear Common Dreams reader, The U.S. is on a fast track to authoritarianism like nothing I've ever seen. Meanwhile, corporate news outlets are utterly capitulating to Trump, twisting their coverage to avoid drawing his ire while lining up to stuff cash in his pockets. That's why I believe that Common Dreams is doing the best and most consequential reporting that we've ever done. Our small but mighty team is a progressive reporting powerhouse, covering the news every day that the corporate media never will. Our mission has always been simple: To inform. To inspire. And to ignite change for the common good. Now here's the key piece that I want all our readers to understand: None of this would be possible without your financial support. That's not just some fundraising cliche. It's the absolute and literal truth. We don't accept corporate advertising and never will. We don't have a paywall because we don't think people should be blocked from critical news based on their ability to pay. Everything we do is funded by the donations of readers like you. Will you donate now to help power the nonprofit, independent reporting of Common Dreams? Thank you for being a vital member of our community. Together, we can keep independent journalism alive when it’s needed most. - Craig Brown, Co-founder |
Two days after President Joe Biden's administration rejected a petition asking federal regulators to use their authority to lower the astronomical price of a lifesaving prostate cancer drug developed entirely with public funds, petitioners on Thursday filed an administrative appeal.
At issue is enzalutamide, a drug the Japanese pharmaceutical giant Astellas and its U.S. counterpart Pfizer sell under the brand name Xtandi. Although Xtandi owes its existence to U.S. taxpayers, who bankrolled 100% of its development, an annual supply of the drug costs $189,900 in the United States—three to six times more than its list price in other wealthy nations.
In late 2021, prostate cancer patients Robert Sachs, Clare Love, and Eric Sawyer petitioned the U.S. Department of Health and Human Services (HHS) to exercise its "march-in rights" against Xtandi. Under the Bayh-Dole Act, the federal government can reclaim and redistribute patents for inventions created with public funding—enabling generic competitors to produce cheaper versions—when "action is necessary to alleviate health or safety needs" or when an invention's benefits are not being made "available to the public on reasonable terms."
HHS Secretary Xavier Becerra referred the petition to the National Institutes of Health (NIH), whose acting Director Lawrence Tabak argued in a Tuesday letter that "Xtandi is widely available to the public on the market," citing Astellas' estimate that "more than 200,000 patients were treated with Xtandi from 2012 to 2021."
Even with insurance, co-pays for Xtandi are sky-high. Medicare recipients, for example, are expected to pay roughly $10,000 per year for the medicine. Especially for the millions of uninsured and underinsured people in the U.S., Xtandi remains completely out of reach.
Tabak's letter went on to say that Xtandi's "practical application is evidenced by the 'manufacture, practice, and operation' of the invention and the invention's 'availability to and use by the public….'" As Knowledge Ecology International executive director James Love lamented, the NIH completely elided any mention of "reasonable terms," editing out that key phrase from Bayh-Dole.
In their appeal, the petitioners wrote: "The petition focused on a single issue: the reasonableness of charging U.S. cancer patients three to six times more than residents of other high-income countries for the drug Xtandi."
"There is no dispute about the following facts," the appeal continues. "Xtandi was invented on grants from the U.S. Army and the NIH at UCLA, a public university. The patents were licensed eventually to Astellas, a Japanese drug company, with a partnership share now held by Pfizer, following its 2016 $14 billion acquisition of Medivation, UCLA's original licensee, that occurred just after the NIH rejected an earlier march-in request on Xtandi. The prices in the United States have consistently been far higher than the prices in other high-income countries."
Prior to the 2021 petition, Clare Love and prostate cancer patient David Reed filed a petition, later joined by Sachs, with the U.S. Department of Defense (DOD) after the Senate Armed Services Committee instructed the Pentagon to initiate march-in proceedings when the price of a drug created with a DOD grant exceeds the median price in seven large high-income nations. The Pentagon, however, has yet to acknowledge or act on the petition submitted to it in February 2019.
"If you consider both of these requests together, a petition to exercise the government's march-in or other rights in the Xtandi patents has been pending before the federal government for more than four years," Thursday's appeal states. "The HHS petition was filed 16 months ago."
It continues:
The petitions were filed with the DOD and HHS instead of the NIH because the NIH has repeatedly demonstrated its unwillingness to even acknowledge that the Bayh-Dole Act includes an obligation to make products invented with federal funds 'available to the public on reasonable terms.' This is demonstrated by a track record of dismissing multiple requests to use the government's Bayh-Dole safeguard to address pricing abuses and access restrictions, including those concerning the federal government's march-in rights under 35 USC § 203, and the federal government's global royalty-free license, under 35 USC § 202(c)(4). There are also extensive email records between Mark Rohrbaugh, currently NIH special adviser for technology transfer who is a long-time agency official, and lobbyists for drug companies and university rights holders, obtained through Freedom of Information Act requests, which not only express opposition to any safeguards regarding unreasonable pricing but organize public relations efforts against using a march-in request to address the pricing of products.
"HHS chose to assign to the NIH the evaluation of our petition regarding Xtandi," says the appeal. "We request HHS to consider this appeal directly, and not assign NIH to review its own decision. The latter would be tantamount to no review at all."
Since Bayh-Dole was enacted in 1980, "march-in rights have never been used... and NIH has repeatedly rejected the idea that affordability is a reasonable term," The American Prospect reported Wednesday. With Xtandi, "advocates thought they found the perfect test case for a new administration that paid lip service to lowering prescription drug costs."
As The Lever noted on Wednesday, the NIH's decision this week was consistent with Biden's track record:
Biden was vice president when the Obama administration rejected congressional Democrats' demand that the government use the same power to lower the skyrocketing prices of medicine in America.
As a senator in 2000, Biden was one of just eight Democrats who helped pharmaceutical lobbyists kill a measure spearheaded by Sen. Paul Wellstone (D-Minn.) and then-Rep. Bernie Sanders (I-Vt.) that would have reinstated the Reagan-era requirement that drug companies sell medicines developed with public money at a reasonable price.
That requirement was repealed by the Clinton administration in 1995, following pressure by drugmakers.
But Becerra's acquiescence to Big Pharma was more surprising. Prior to joining the Biden administration, the HHS secretary had expressed support for wielding the executive branch's authority to rein in soaring drug prices.
As the attorney general of California in the summer of 2020, "Becerra demanded the Trump administration use existing law to lower the price of medicines that were originally developed at taxpayer expense," The Lever reported. "As a member of Congress in 2016, Becerra signed on to a letter to the Obama Department of Health and Human Services calling on officials to broadly use 'march-in rights' to lower the cost of prescription drugs—including 'specialty drugs, like those to treat cancer, which are frequently developed with taxpayer funds.'"
Despite pressure from numerous members of Congress and medicine affordability advocacy groups, the NIH declared Tuesday that it "does not believe that use of the march-in authority would be an effective means of lowering the price of the drug."
Instead, the agency vowed to "pursue a whole-of-government approach informed by public input to ensure the use of march-in authority is consistent with the policy and objective of the Bayh-Dole Act," a move that progressive advocates denounced as a "pathetic" attempt to deflect criticism of its failure to use or threaten to use its legal power.
“This is a drug that was invented with taxpayer dollars by scientists at UCLA and can be purchased in Canada for one-fifth the U.S. price," Sanders said Tuesday. "The Japanese drugmaker Astellas, which made $1 billion in profits in 2021, has raised the price of this drug by more than 75%."
"How many prostate cancer patients will die because they cannot afford this unacceptable price?" asked Sanders, chair of the Senate Committee on Health, Education, Labor, and Pensions.
During a Wednesday hearing, Sanders made the case for changing "the current culture of greed into a culture which understands that science and medical breakthroughs should work for ordinary people, and not just enrich large corporations and CEOs."
Two days after President Joe Biden's administration rejected a petition asking federal regulators to use their authority to lower the astronomical price of a lifesaving prostate cancer drug developed entirely with public funds, petitioners on Thursday filed an administrative appeal.
At issue is enzalutamide, a drug the Japanese pharmaceutical giant Astellas and its U.S. counterpart Pfizer sell under the brand name Xtandi. Although Xtandi owes its existence to U.S. taxpayers, who bankrolled 100% of its development, an annual supply of the drug costs $189,900 in the United States—three to six times more than its list price in other wealthy nations.
In late 2021, prostate cancer patients Robert Sachs, Clare Love, and Eric Sawyer petitioned the U.S. Department of Health and Human Services (HHS) to exercise its "march-in rights" against Xtandi. Under the Bayh-Dole Act, the federal government can reclaim and redistribute patents for inventions created with public funding—enabling generic competitors to produce cheaper versions—when "action is necessary to alleviate health or safety needs" or when an invention's benefits are not being made "available to the public on reasonable terms."
HHS Secretary Xavier Becerra referred the petition to the National Institutes of Health (NIH), whose acting Director Lawrence Tabak argued in a Tuesday letter that "Xtandi is widely available to the public on the market," citing Astellas' estimate that "more than 200,000 patients were treated with Xtandi from 2012 to 2021."
Even with insurance, co-pays for Xtandi are sky-high. Medicare recipients, for example, are expected to pay roughly $10,000 per year for the medicine. Especially for the millions of uninsured and underinsured people in the U.S., Xtandi remains completely out of reach.
Tabak's letter went on to say that Xtandi's "practical application is evidenced by the 'manufacture, practice, and operation' of the invention and the invention's 'availability to and use by the public….'" As Knowledge Ecology International executive director James Love lamented, the NIH completely elided any mention of "reasonable terms," editing out that key phrase from Bayh-Dole.
In their appeal, the petitioners wrote: "The petition focused on a single issue: the reasonableness of charging U.S. cancer patients three to six times more than residents of other high-income countries for the drug Xtandi."
"There is no dispute about the following facts," the appeal continues. "Xtandi was invented on grants from the U.S. Army and the NIH at UCLA, a public university. The patents were licensed eventually to Astellas, a Japanese drug company, with a partnership share now held by Pfizer, following its 2016 $14 billion acquisition of Medivation, UCLA's original licensee, that occurred just after the NIH rejected an earlier march-in request on Xtandi. The prices in the United States have consistently been far higher than the prices in other high-income countries."
Prior to the 2021 petition, Clare Love and prostate cancer patient David Reed filed a petition, later joined by Sachs, with the U.S. Department of Defense (DOD) after the Senate Armed Services Committee instructed the Pentagon to initiate march-in proceedings when the price of a drug created with a DOD grant exceeds the median price in seven large high-income nations. The Pentagon, however, has yet to acknowledge or act on the petition submitted to it in February 2019.
"If you consider both of these requests together, a petition to exercise the government's march-in or other rights in the Xtandi patents has been pending before the federal government for more than four years," Thursday's appeal states. "The HHS petition was filed 16 months ago."
It continues:
The petitions were filed with the DOD and HHS instead of the NIH because the NIH has repeatedly demonstrated its unwillingness to even acknowledge that the Bayh-Dole Act includes an obligation to make products invented with federal funds 'available to the public on reasonable terms.' This is demonstrated by a track record of dismissing multiple requests to use the government's Bayh-Dole safeguard to address pricing abuses and access restrictions, including those concerning the federal government's march-in rights under 35 USC § 203, and the federal government's global royalty-free license, under 35 USC § 202(c)(4). There are also extensive email records between Mark Rohrbaugh, currently NIH special adviser for technology transfer who is a long-time agency official, and lobbyists for drug companies and university rights holders, obtained through Freedom of Information Act requests, which not only express opposition to any safeguards regarding unreasonable pricing but organize public relations efforts against using a march-in request to address the pricing of products.
"HHS chose to assign to the NIH the evaluation of our petition regarding Xtandi," says the appeal. "We request HHS to consider this appeal directly, and not assign NIH to review its own decision. The latter would be tantamount to no review at all."
Since Bayh-Dole was enacted in 1980, "march-in rights have never been used... and NIH has repeatedly rejected the idea that affordability is a reasonable term," The American Prospect reported Wednesday. With Xtandi, "advocates thought they found the perfect test case for a new administration that paid lip service to lowering prescription drug costs."
As The Lever noted on Wednesday, the NIH's decision this week was consistent with Biden's track record:
Biden was vice president when the Obama administration rejected congressional Democrats' demand that the government use the same power to lower the skyrocketing prices of medicine in America.
As a senator in 2000, Biden was one of just eight Democrats who helped pharmaceutical lobbyists kill a measure spearheaded by Sen. Paul Wellstone (D-Minn.) and then-Rep. Bernie Sanders (I-Vt.) that would have reinstated the Reagan-era requirement that drug companies sell medicines developed with public money at a reasonable price.
That requirement was repealed by the Clinton administration in 1995, following pressure by drugmakers.
But Becerra's acquiescence to Big Pharma was more surprising. Prior to joining the Biden administration, the HHS secretary had expressed support for wielding the executive branch's authority to rein in soaring drug prices.
As the attorney general of California in the summer of 2020, "Becerra demanded the Trump administration use existing law to lower the price of medicines that were originally developed at taxpayer expense," The Lever reported. "As a member of Congress in 2016, Becerra signed on to a letter to the Obama Department of Health and Human Services calling on officials to broadly use 'march-in rights' to lower the cost of prescription drugs—including 'specialty drugs, like those to treat cancer, which are frequently developed with taxpayer funds.'"
Despite pressure from numerous members of Congress and medicine affordability advocacy groups, the NIH declared Tuesday that it "does not believe that use of the march-in authority would be an effective means of lowering the price of the drug."
Instead, the agency vowed to "pursue a whole-of-government approach informed by public input to ensure the use of march-in authority is consistent with the policy and objective of the Bayh-Dole Act," a move that progressive advocates denounced as a "pathetic" attempt to deflect criticism of its failure to use or threaten to use its legal power.
“This is a drug that was invented with taxpayer dollars by scientists at UCLA and can be purchased in Canada for one-fifth the U.S. price," Sanders said Tuesday. "The Japanese drugmaker Astellas, which made $1 billion in profits in 2021, has raised the price of this drug by more than 75%."
"How many prostate cancer patients will die because they cannot afford this unacceptable price?" asked Sanders, chair of the Senate Committee on Health, Education, Labor, and Pensions.
During a Wednesday hearing, Sanders made the case for changing "the current culture of greed into a culture which understands that science and medical breakthroughs should work for ordinary people, and not just enrich large corporations and CEOs."