May, 23 2013, 09:07am EDT

For Immediate Release
Contact:
Nasima Hossain, U.S. PIRG
Work: 202-461-3826, Cell: 202-256-8419
nhossain@pirg.org
New Report Documents a Decade of Safety Violations by Compounding Pharmacies
The contaminated drug that caused last fall's fungal meningitis outbreak and killed 55 people is just the tip of the iceberg of an industry-wide problem, according to a new report released today by U.S. PIRG. The meningitis outbreak was simply the latest and deadliest in a long line of errors and risky practices by compounding pharmacies.
WASHINGTON
The contaminated drug that caused last fall's fungal meningitis outbreak and killed 55 people is just the tip of the iceberg of an industry-wide problem, according to a new report released today by U.S. PIRG. The meningitis outbreak was simply the latest and deadliest in a long line of errors and risky practices by compounding pharmacies.
"Consumers should always be confident that their drugs are safe and effective, regardless of whether their drugs are manufactured at a compounding facility or a pharmaceutical company. Our prescription drugs should not lead to the illness and death of our loved ones," said Nasima Hossain, Public Health Advocate for U.S. PIRG.
Traditionally, compounding pharmacies have engaged in the practice of customizing a medication for a particular patient - such as altering the dosage or turning a pill into a liquid for patients who have difficulty swallowing. But now, large compounding pharmacies are behaving exactly like drug manufacturers. Although they manufacture drugs in bulk, large compounding pharmacies do not conduct rigorous testing, nor do they adhere to safe manufacturing processes that pharmaceutical companies are required to implement. Instead they are exploiting legal loopholes in the law to escape the necessary safety standards and oversight.
The report, "Prescription for Danger," analyzed more than 40 warning letters issued by the Food and Drug Administration (FDA) to compounding pharmacies from January 2002 to December 2012. Each firm was cited for multiple violations of the Food, Drug, and Cosmetic Act, such as making new drugs that have not been tested for safety and effectiveness, and making drugs in unsanitary conditions.
"As the U.S. PIRG report accurately points out, this 'shadow industry' of compounding pharmacies recklessly puts Americans' lives at risk by ignoring basic standards for safety and cleanliness when performing large-scale compounding operations," said U.S. Senator Richard Blumenthal (CT). "I hope the FDA will assert its full authority over large-scale compounders to protect the public from unsafe and untested drug products. These pharmacies currently exist in a legal netherworld - outside the bounds of state and federal oversight - and need to be heavily regulated by the agency."
"This report gives a clearer picture of what has been going on at compounding pharmacies. Although the FDA didn't do enough, they documented major violations involving different types of unsafe drugs, not just contaminated ones," said Diana Zuckerman, President of the National Research Center for Women & Families Cancer Prevention and Treatment Fund. "This shows that Congress needs to make major improvements in the law to protect all types of patients - those with cancer, heart disease, diabetes, asthma, eye diseases, anything that requires safe and effective medications."
"Congress must give the FDA the authority it needs to ensure that drugs made in compounding pharmacies are safe," said Hossain. "We must never repeat the avoidable tragedy of having contaminated and unsafe drugs on the market again."
The report highlights some of the most blatant violations by compounding pharmacies, including:
* In 2002, consumers complained about arthritis pain relief injections produced by Lee Pharmacy in Fort Smith, Arkansas. The FDA analyzed the injections and found they were contaminated with penicillium rugulosum, a potentially lethal fungus.
* In 2009, Hopewell Pharmacy in Hopewell, New Jersey, was found to be using a solvent called diethylene glycol monoethyl ether in sterile injections used for the treatment of varicose veins. This ingredient is normally used in industrial cleaners and is not approved by the FDA for use in drug manufacturing.
* In 2005, University Pharmacy in Salt Lake City, Utah, was investigated because a 25-year-old woman lapsed into a coma and died from using Photocaine, a topical anesthetic cream produced by the pharmacy without the approval of the FDA.
U.S. PIRG, the federation of state Public Interest Research Groups (PIRGs), stands up to powerful special interests on behalf of the American public, working to win concrete results for our health and our well-being. With a strong network of researchers, advocates, organizers and students in state capitols across the country, we take on the special interests on issues, such as product safety,political corruption, prescription drugs and voting rights,where these interests stand in the way of reform and progress.
LATEST NEWS
Led by US, Global Military Spending Surged to Record $2.4 Trillion Last Year
"Can we get some healthcare please, or maybe feed some of the 40 million+ Americans who can't get enough food?" asked the watchdog group Public Citizen.
Apr 22, 2024
New research published Monday shows that global military spending increased in 2023 for the ninth consecutive year, surging to $2.4 trillion as Russia's assault on Ukraine and Israel's war on the Gaza Strip helped push war-related outlays to an all-time high.
The Stockholm International Peace Research Institute (SIPRI) recorded military spending increases in every geographical region it examined last year, from Europe to Oceania to the Middle East. Last year's global increase of 6.8% was the largest since 2009, SIPRI said.
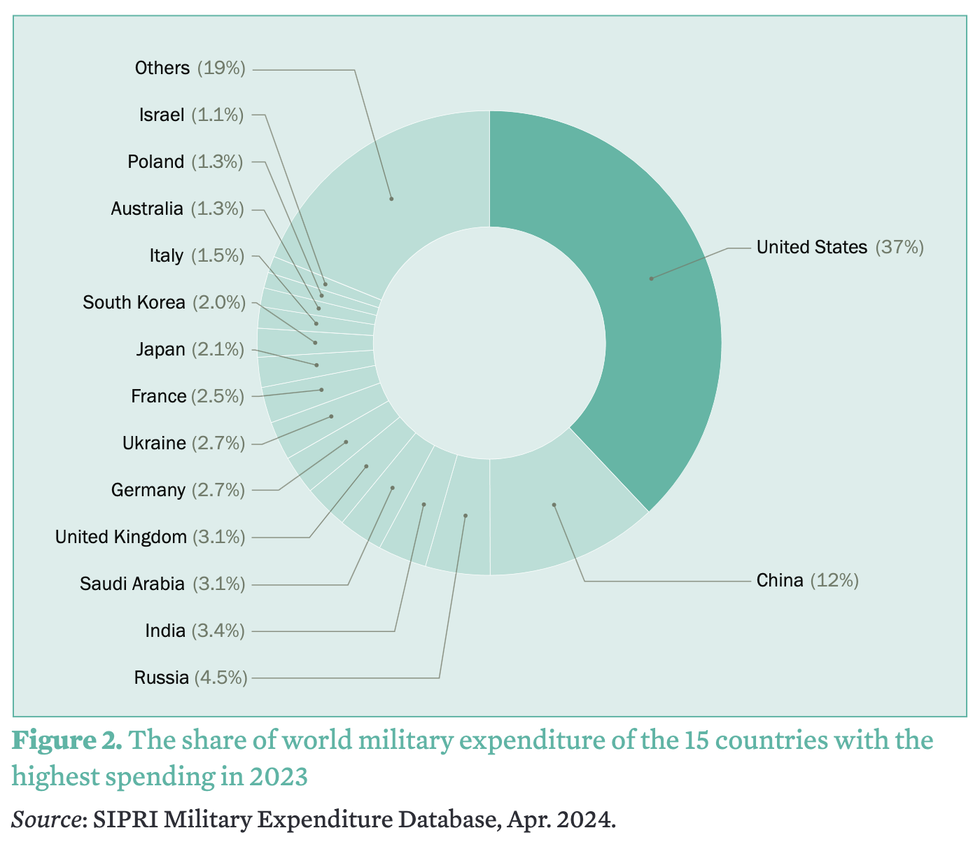
The United States was by far the largest military spender at $916 billion in 2023, up 2.3% compared to the previous year. The next biggest spender was China, which poured an estimated $296 billion into its military last year—three times less than the U.S.
"Can we get some healthcare please, or maybe feed some of the 40 million+ Americans who can't get enough food?" asked the watchdog group Public Citizen in response to SIPRI's report, which found that the U.S. accounted for 37% of the world's total military spending last year.
A separate analysis of U.S. military spending in 2023 found that 62% of the country's federal discretionary budget went to militarized programs, leaving less than half of the budget for healthcare, housing, nutrition assistance, education, and other domestic priorities.
Together, SIPRI found, the top five biggest military spenders last year—the U.S., China, Russia, India, and Saudi Arabia—accounted for 61% of global military outlays.
"The unprecedented rise in military spending is a direct response to the global deterioration in peace and security," Nan Tian, senior researcher with SIPRI's Military Expenditure and Arms Production Program, said in a statement. "States are prioritizing military strength but they risk an action-reaction spiral in the increasingly volatile geopolitical and security landscape."
In the Middle East, military spending jumped by 9% last year—the highest annual growth rate in the past decade. Israel, which relies heavily on weapons imports from the U.S., spent 24% more on its military last year than in 2022, according to SIPRI, an increase fueled by the country's devastating assault on Gaza.
SIPRI found that NATO's 31 member countries dumped a combined $1.3 trillion into military expenditures in 2023, accounting for 55% of the global total.
U.S. military spending, which is poised to continue surging in the coming years, made up 68% of NATO's 2023 total.
Keep ReadingShow Less
IDF Kills 18 Children in Rafah Hours After US House Approves Billions in Military Aid
"Members of Congress should understand that approving more military aid could subject them to personal liability for aiding and abetting an ongoing genocide in Gaza."
Apr 22, 2024
Hours after the U.S. House approved legislation that would send billions of dollars in additional military aid to Israel, the country's forces killed nearly two dozen people in Rafah, the southern Gaza city where more than half of the enclave's population is sheltering.
Gaza health officials said Sunday that the weekend strikes on Rafah—a former "safe zone" that Israel has been threatening to invade for weeks—killed 22 people, including 18 children. The Associated Pressreported that the first of the Israeli strikes "killed a man, his wife, and their 3-year-old child, according to the nearby Kuwaiti Hospital, which received the bodies."
"The woman was pregnant and the doctors saved the baby, the hospital said," AP added. "The second strike killed 17 children and two women from an extended family."
Israeli forces have killed more than 14,000 children in Gaza since October, but the Biden administration and American lawmakers have refused to back growing international calls to cut off the supply of weaponry and other military equipment even as U.S. voters express support for an arms embargo.
The measure the House approved on Saturday includes $26 billion in funding for Israel, much of which is military assistance.
"Just a day after the House voted to send $14 billion in unconditional military funding to [Israeli Prime Minister Benjamin] Netanyahu's campaign of death and destruction, he bombed the safe zone of Rafah AGAIN, killing 22 Palestinians, of which 18 were CHILDREN!" U.S. Rep. Delia Ramirez (D-Ill.), one of the 58 House lawmakers who voted against the legislation, wrote on social media late Sunday.
"History books will write about today and the past seven months, and how our nation's leaders lacked the courage and moral clarity to stand up to a tyrant," she added. "Shameful."
The military aid package for Israel now heads to the U.S. Senate, which is set to consider the bill early this week. U.S. President Joe Biden, who has continued to greenlight arms sales to Israel amid clear evidence of war crimes, is expected to sign the measure if it reaches his desk.
"Rather than sending more weapons to Israel, Congress should declare an immediate arms embargo on Israel."
U.S. law prohibits "arms transfers that risk facilitating or otherwise contributing to violations of human rights or international humanitarian law," according to a White House memo issued in February. The U.S. State Department has said repeatedly that it has not found Israel to be in violation of international law, a position that runs directly counter to the findings of leading humanitarian organizations and United Nations experts.
The investigative outlet ProPublicareported last week that a "special State Department panel recommended months ago that Secretary of State Antony Blinken disqualify multiple Israeli military and police units from receiving U.S. aid after reviewing allegations that they committed serious human rights abuses" prior to the October 7 Hamas-led attack on southern Israel.
"But Blinken has failed to act on the proposal in the face of growing international criticism of the Israeli military's conduct in Gaza, according to current and former State Department officials," ProPublica noted.
Sarah Leah Whitson, executive director of Democracy for the Arab World Now (DAWN), said in a statement Sunday that senators "should reject sending additional weapons to Israel not only because our laws prohibit military aid to abusive regimes, but because it's extremely damaging to our national interests."
DAWN's advocacy director, Raed Jarrar, added that "at a time when Israel is bracing for International Criminal Court arrest warrants against its leaders, members of Congress should understand that approving more military aid could subject them to personal liability for aiding and abetting an ongoing genocide in Gaza."
"Rather than sending more weapons to Israel," said Jarrar, "Congress should declare an immediate arms embargo on Israel."
Keep ReadingShow Less
Ahead of Treaty Negotiations, Hundreds March to 'End the Plastic Era'
"As adults who come to Ottawa to negotiate the plastic treaty, you must protect our rights to live in a healthy and safe environment," one young activists said.
Apr 21, 2024
Days before national delegates gather for the fourth and penultimate negotiations to develop a Global Plastics Treaty in Ottawa, Canada, around 500 Indigenous and community representatives, members of civil society and environmental groups, and experts and scientists gathered for a "March to End the Plastic Era" on Sunday.
The protesters, organized under the banner of Break Free From Plastic, called for a treaty that significantly reduces plastic production and centers the frontline communities most impacted by the plastics crisis.
"Delegates must act like our lives depend on it—because they do," Daniela Duran Gonzales, senior legal campaigner with the Center for International Environmental Law, said in a statement. "Our climate goals, the protection of human health, the enjoyment of human rights, and the rights of future generations all rest on whether the future plastics treaty will control and reduce polymers to successfully end the plastic pollution crisis."
"Short-sighted business interests must be out of the room because the only way to achieve equitable livelihoods is when we have a healthy planet."
The official meeting of the Intergovernmental Negotiating Committee (INC) to craft a "international legally binding instrument on plastic pollution, including in the marine environment," will run from April 23 to 29 in the Canadian capital.
Break Free From Plastic called the negotiations a "make or break" moment for the treaty, which is supposed to be completed in late 2024 in Busan, South Korean. However, civil society groups have expressed concern that oil-producing countries and the plastics industry will water down the agreement and steer it toward waste management and recycling, which has been revealed to be a false solution to plastic pollution knowingly promoted by the industry for decades.
The last round of negotiations concluded in late 2023 in Nairobi, Kenya, with little progress made after 143 fossil fuel and chemical lobbyists attended.
Salisa Traipipitsiriwat of Thailand, who is the senior campaigner and Southeast Asia plastics project manager for the Environmental Justice Foundation, said ahead of Sunday's march that it was "crucial for world leaders to step up and put the people and planet at the forefront."
"Short-sighted business interests must be out of the room because the only way to achieve equitable livelihoods is when we have a healthy planet," Traipipitsiriwat added.
On Sunday, marchers gathered for a press conference at 10:30 am ET before marching at around 11:30 am from Parliament Hill to the Shaw Center, were negotiations will begin on Tuesday. Crowds began to disperse around 1:30 pm. Participants carried large banners with messages including, "End the plastic era," "End multigenerational toxic exposure," and pointing out that 99% of plastics came from fossil fuels. The gathering featured live music and art, including a giant tap pouring out plastics and a "Plastisaurus rex" with the message "Make single-use plastic extinct."

"Now's the time to be bold and push for a treaty that cuts plastic production and holds polluters accountable," Julie Teel Simmonds, a senior attorney at the Center for Biological Diversity, said in a pre-march statement. "I'm inspired to be joining so many advocates in Ottawa, standing up against the enormous harm the fossil fuel and petrochemical industries are causing to people's health and the planet. I hope to see countries showing ambition this week, and I urge them to remember what's at stake for future generations."
Civil society groups have compiled several demands for an ambitious and effective treaty. These are:
- Centering human rights, especially those of Indigenous communities, young people, and workers most impacted by plastic pollution;
- Protecting the rights of Indigenous peoples throughout the treaty process;
- Dealing with plastics across their entire lifecyle;
- Reducing production as a "nonnegotiable" part of the treaty;
- Eliminating toxic chemicals and additives from plastics;
- Bolstering reuse systems for plastics that are non-toxic;
- Prioritizing first prevention, then reuse, recycling, recovery, and disposal when managing plastic waste;
- Ending "waste colonialism" by strengthening regulations for trading plastics;
- Guaranteeing a "just transition" for people employed across the plastics lifecycle;
- Including "non-party" provisions in the treaty;
- Establishing a mechanism to fund countries so they can fully implement the treaty; and
- Enshrining conflict-of-interest policies as a protection against plastics industry lobbying.
The coalition emphasized the need to tackle the problem of plastic from cradle to grave.
"Plastic doesn't just become pollution when it's thrown away," said Jessica Roff, the U.S. and Canada plastics and petrochemicals program manager for the Global Alliance for Incinerator Alternatives. "Plastic is pollution, from the moment the fossil fuels are extracted from the ground to the eternity of waste it spawns."
Chrie Wilke, global advocacy manager for the Waterkeeper Alliance, said "Clearly the crux of the plastic pollution crisis is too much plastic being produced. There is no way to recycle our way out of this. We must face the fact that plastic and petrochemicals, at current production levels, endanger waterways, communities, and fisheries across the globe. Cutting production and implementing non-plastic alternatives and reuse systems is essential."

Activists also emphasized the environmental justice implications of plastic pollution, and how some communities and groups are more burdened than others, both from the dangers of the production process and from waste disposal.
"Children and youth like me suffer the most and are recognized as a vulnerable group," said Aeshnina 'Nina' Azzahra, the founder of River Warrior Indonesia. "My playground and my future are at risk. We all want our environment to be plastic-free, but please don't put your burden on the other side of the world—this is NOT fair. As adults who come to Ottawa to negotiate the plastic treaty, you must protect our rights to live in a healthy and safe environment."
Jo Banner, co-founder and co-directer of The Descendants Project, said:"Frontline community members, such as myself, are participating in these treaty negotiations with heavy hearts as our communities back home are struggling with sickness and disease caused by the upstream production of plastic."
"Although our hearts are heavy, they are full with passion urging negotiators to aim for an ambitious treaty that caps plastic production," Banner added. "Areas such as my hometown, located in the heart of Louisiana's Cancer Alley, need a strong treaty now. There is no more time to waste."
Keep ReadingShow Less
Most Popular